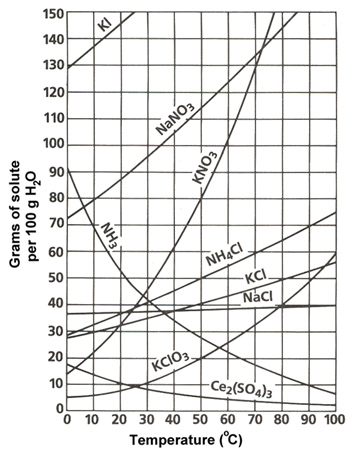

A. 30 grams
Incorrect. At 70°C, 130 grams of KNO3
is dissolved. At 40°C, 60 grams of KNO3 is dissolved. Subtract 130 grams
from 40 grams to find out the amount of KNO3 that will crystallize
out.
B. 55 grams
Incorrect. At 70°C, 130 grams of KNO3
is dissolved. At 40°C, 60 grams of KNO3 is dissolved. Subtract 130 grams
from 40 grams to find out the amount of KNO3 that will crystallize
out.
C. 60 grams
Incorrect. At 70°C, 130 grams of KNO3
is dissolved. At 40°C, 60 grams of KNO3 is dissolved. Subtract 130 grams
from 40 grams to find out the amount of KNO3 that will crystallize
out.
D. 70 grams
Correct!
A student needs to fully dissolve 30 grams of KClO3 in 100 grams of H2O. What is the lowest possible temperature the solution can be heated to fully dissolve all of this solute?
A. 10°C
Incorrect. Find 30 grams on the y-axis and follow it until it intersects with the KClO3 line. Then follow that point down to the x-axis to determine what temperature the solution needs to dissolve exactly 30 grams of KClO3.
B. 35°C
Incorrect. Find 30 grams on the y-axis and follow it until it intersects with the KClO3 line. Then follow that point down to the x-axis to determine what temperature the solution needs to dissolve exactly 30 grams of KClO3.
C. 55°C
Incorrect. Find 30 grams on the y-axis and follow it until it intersects with the KClO3 line. Then follow that point down to the x-axis to determine what temperature the solution needs to dissolve exactly 30 grams of KClO3.
D. 70°C
Correct!

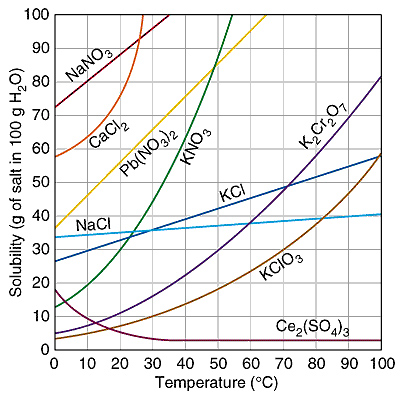
A. 80 grams
Incorrect. Find 50°C on the x-axis and follow it
up until it intersects with the line for K2Cr2O7.
Then follow over to the y-axis to see the amount that can be dissolved at 50°C.
B. 65 grams
Incorrect. Find 50°C on the x-axis and follow it
up until it intersects with the line for K2Cr2O7.
Then follow over to the y-axis to see the amount that can be dissolved at 50°C.
C. 50 grams
Incorrect. Find 50°C on the x-axis and follow it
up until it intersects with the line for K2Cr2O7.
Then follow over to the y-axis to see the amount that can be dissolved at 50°C.
D. 30 grams
Correct!

A. It will dissolve because it is an unsaturated solution.
Correct!
B. It will dissolve because it is a supersaturated solution.
Incorrect. At 30°C, NaNO3 can dissolve about 95 grams. The question asks, 55 grams + 5 grams = 60 grams. The total amount is still below 95 grams, so this is an unsaturated solution where all solute will fully dissolve.
C. It will not dissolve because it is a saturated solution.
Incorrect. At 30°C, NaNO3 can dissolve about 95 grams. The question asks, 55 grams + 5 grams = 60 grams. The total amount is still below 95 grams, so this is an unsaturated solution where all solute will fully dissolve.
D. Not enough information is given to determine what will happen.
Incorrect. At 30°C, NaNO3 can dissolve about 95 grams. The question asks, 55 grams + 5 grams = 60 grams. The total amount is still below 95 grams, so this is an unsaturated solution where all solute will fully dissolve.